The limit of viability
Rescue lives, cure diseases, alleviate symptoms – that is what modern medications do a million times over, day after day. But we are far from having a medicine for every complaint. Moreover, corona has made us keenly aware that new illnesses are constantly joining those which are already known. New medications are, thus, constantly needed, and development work on them is ongoing. These medicines should, of course, be both effective as well as safe – ideally even before they are tested on humans. This last phase – the clinical trial phase – is essential. However, a great deal about the effect of a pharmaceutical product can also be determined already in the lab. Because this effect unfolds largely in the cells of the human body. As a result, a cell culture can serve as a substitute for "trying out" the medication.
“We can, for example, determine the limit value above which a substance becomes toxic to the cells,” explains Márton Nagy, biotechnology developer at the Munich-based company INCYTOИ ®. “This applies not only for medications, by the way, but also for, e.g., potential environmental toxins. We place a certain quantity of the substance in the nutrient solution that contains the culture and observe how the cells respond. The quantity is then increased incrementally.
Based on certain measurement data and the optical monitoring with a microscope, we can identify the point at which it becomes critical for the cells. This value can be converted to the body weight of a person. In practice, the permissible limit value for a dose is then generally defined as a fraction of this critical value.” In pharmaceutical research, many tests are performed using cancer cells. With these cells, however, the tables are turned: here, the objective is to determine which medication, and in what quantity inhibits their reproduction or completely destroys them.
The observation of the cells is a multilayer and, above all, relatively time-consuming process. A single trial takes an average of approximately three days. During this period, numerous individual measurements are performed, and the cells are repeatedly photographed in short intervals. The image sequences can be merged together to create a time-lapse film that shows the course of the cell growth. Three physical quantities are examined for the measurements: oxygen content, pH value and electrical resistance of the cell layer.
Physical quantities provide information
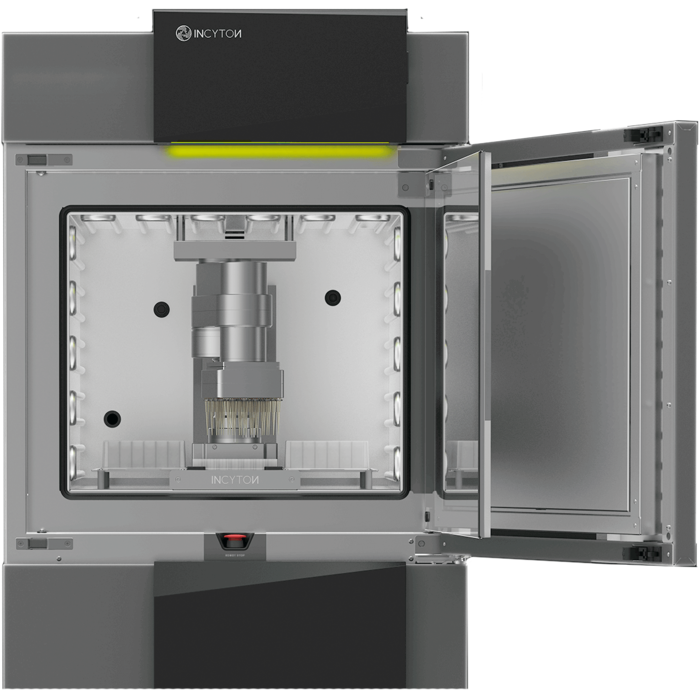
Up until now, such test series required a great deal of manual work. The various steps could only be partially automated. With CYRIS ®FLOX, INCYTOИ ® has created a fully automatic device that can perform the multi-day test run without human intervention and can also fully document the results.
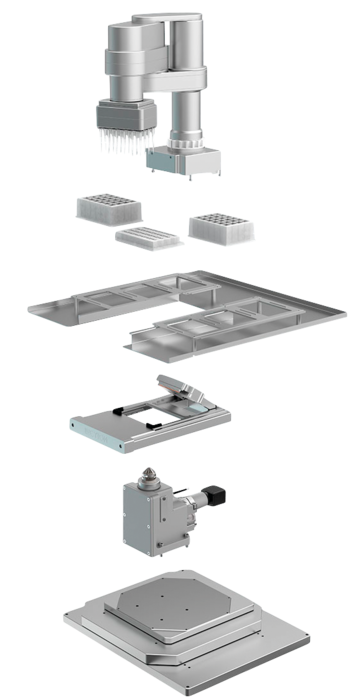
At the heart of the test setup is a microtitre plate made of transparent material that has 24 wells, or test chambers. These hold the cell samples like miniature Petri dishes. 24 pipettes on a robot arm supply the small cultures with nutrient solution and feed in the substances that are to be tested. In doing so, a different composition of the solution can be selected for each pipette. Each test chamber is equipped with sensors for oxygen content, pH value and electrical resistance. The individual test chambers are photographed from below at regular intervals through a microscope lens.
Development for automation
INCYTOИ ® is a start-up with academic roots. The founders of the company worked previously in university research. There, they used motors from other manufacturers for the initial prototypes of their device. These did not prove to be suitable and were later replaced by models from FAULHABER. They convinced with their compact design and the reliability of the components. When it came to the further development of the system to prepare for series production, there was, thus, no need to find a drive supplier. New goals were, however, defined for this area: “We wanted to work with as few different motor types as possible,” says Matthias Moll, head of development, describing the initial situation. “We also wanted a simpler arrangement for the wiring. We were looking for a drive in which the electronics are already integrated. Up until then, they were housed in a control element of the robot arm, which meant that many cable connections were necessary in a moving element.” In addition, the motors should be able to report errors, for example, if overheating causes or threatens to cause a mechanical blockage.
In combination with an integrated Motion Controller of the CxD series, the 2232…BX4 brushless servomotor satisfies these new requirements of the technicians from INCYTOИ ® – as well as all others, such as high performance in an extremely compact design, low weight and volume as well as compatibility for laboratory use. Six motors are built into the CYRIS ®FLOX analysis device. Three of these move the pipetting head in the robot arm on three axes. They are responsible for moving the pipettes precisely over the microtitre test chambers and for moving into position just above the chambers for discharging the solution. A fourth motor drives 24 suction pistons, which transport up to 200 µl of culture medium in sterile pipette tips. Two motors move the microscope on an XY table below the cell samples. The photos of the individual test chambers are taken from below through the transparent material of the microtitre plates.
Precision and reliability in continuous operation
“In order to track the development of individual cells in the time lapse later on, the lens must always be at the exact same point under the test chamber,” says Matthias Moll as he explains the challenge in this step. “With the help of the FAULHABER motors, we can precisely position the table to within two micrometres.” For comparison: A human hair has a thickness of between 50 and 70 micrometres. The motor that drives the pistons of the pipette head must also operate very precisely. Only if the quantity of liquid corresponds exactly to the specifications can valid test results be produced.
In CYRIS ®FLOX systems, precision is a continuous task. The maxim for the motor applications is, therefore, repeatability. The exact movement must be performed repeatedly for the days of the test in short intervals without deviation. “We expect the highest possible level of reliability from the drives in continuous operation,” emphasises the head of development. “Only then do we create the conditions for a long 'walk-away time'.” In the world of laboratory automation, this is what one calls the time during which a test can operate without human intervention. “With CYRIS ®FLOX, this time can be extended from a few minutes to hours or days. The highly qualified scientists and laboratory technicians can perform other work in the meantime. The efficiency of the laboratory operations increases, the running costs decrease, and the device quickly pays for itself.”