Finding the head of a pin
"Discovering the mother tumor is normally quite easy and possible through many techniques. But to discover all the metastases, where many are the size of a pinhead, is very difficult," explains Dr. Martin Pärnaste, head engineer in the Cyclotron Systems division of GE Healthcare in the Swedish city of Uppsala. PET helps to detect such metastases. This can be of decisive importance for subsequent therapy.
Like X-ray and computer tomography (CT), PET generates its images with the help of a small dose of radioactive radiation. The radiation is not emitted by a device that directs it at the body from the outside in this case, however. Instead, it comes from radioactive particles that are previously administered to the patient. They are generally mixed with glucose in a so-called radiodiagnostic agent or “tracer” and injected into the bloodstream.
Short half-life
Relatively harmless, low-radiation substances are used for the PET diagnostic agent. They decay rapidly and leave no critical residues. In about 90% of cases, isotope 18F of the halogen Fluorine is used. It has short half-life of approximately 110 minutes, which means that it has lost nearly all of its radioactivity after one day. Other isotopes with a similarly short half-life are used as well.
Because the PET tracers decay so quickly, they cannot be kept in supply like other materials. They must be produced fresh in a particle accelerator – the cyclotron – shortly before they are used. This must not be located too far away from the place of use as every minute counts, even during transport.
Particle race on a spiral path
The first cyclotrons were constructed back in the 1930s by pioneers in particle physics. Their functional principle has since then been modified and further developed numerous times – including the world's largest particle accelerator, CERN, located in Geneva. The technology has also proven itself in medical technology as well, however. To produce isotopes for PET, negatively charged hydrogen ions are accelerated in a vacuum chamber located within the cyclotron. The ions are accelerated by electric fields and kept on a spiral path by a strong magnetic field.
At the end of this path, they fly through a thin graphite foil, thereby losing their electrons and becoming positively charged protons. As a result of this charge reversal, their trajectory changes from the previous spiral movement to a straight line. The orientation of the foil determines the direction of the proton beam. It is directed towards a reaction chamber, the so-called target, in which the source material for the isotope is located. There, the proton beam triggers a nuclear reaction and produces the required isotopes from the target content.
A number of years ago, Dr. Pärnaste and his team were assigned the task of further improving the reaction as well as developing a machine that is as small and economical as possible. It was to contribute to making clinical access to PET isotopes simpler and this imaging technique more widely available. The result of the development was named GENtrace and was launched in 2017 with great success.
Magnet-free drive technology
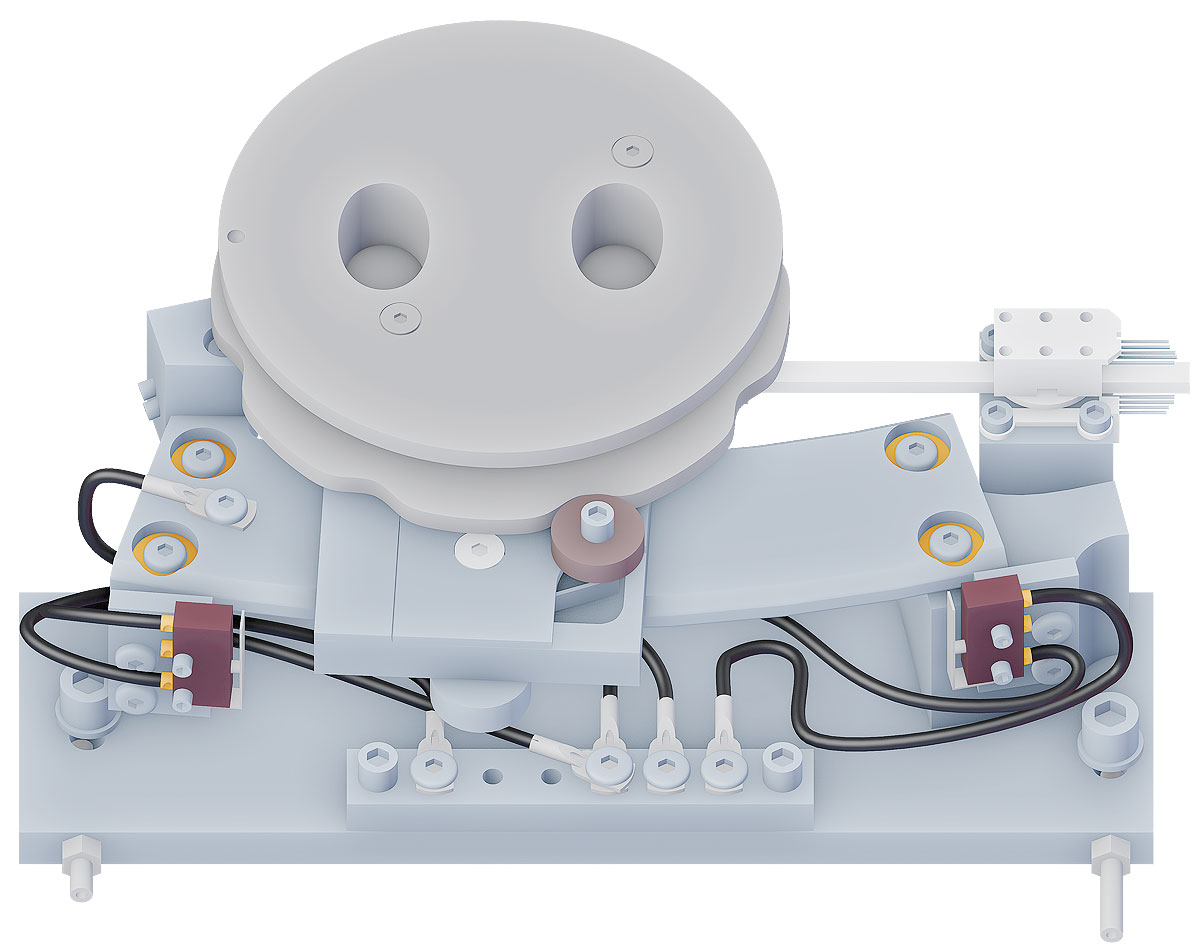
To produce the largest possible quantity of isotopes or isotopes from various elements in a single pass, the new cyclotron has three targets. The orientation of the beam must thus be variable so that it can strike all three targets. To achieve this, the carrier on which the graphite foil is affixed is moved using motor power.
Inside of a cyclotron, however, are conditions with which standard electric motors can hardly cope: magnetic fields, vacuum, electric fields and radiation interfere with their function or make them outright impossible. The motor for directing the beam is therefore normally located outside of the actual cyclotron. Its movement is then transferred to the foil carrier by means of a complex mechanical construction. This has considerable disadvantages, including the mechanical play and the extensive sealing that is required where moving parts pass through the wall of the vacuum chamber.
These disadvantages disappear when using a piezo motor. Its functional principle makes it immune to the inhospitable conditions in the cyclotron. Because, unlike a classic electric motor, it requires neither magnetic components nor rotating parts to convert electric current into movement. Its operating principle is based on the fact that the shape of a piezoceramic element changes when a voltage is applied to it.
Technology from the neighbour
The experts from GE Healthcare became aware of the technology from PiezoMotor through an article in a technical journal. Conveniently, it then turned out that both companies are based in Uppsala. "After testing several micromotors and motion solutions, we finally had a breakthrough in the development. In the final design, we use two drives from PiezoMotor – a 20N linear motor to move the proton beam, and a nonmagnetic rotary motor with 50 mNm torque to adjust the ion extraction," concludes Dr. Pärnaste.
This second drive located within the cyclotron is responsible for positioning the ion source. To extract as many ions as possible with the help of an electrode, the relative position of source and electrode must be repeatedly adjusted. Thanks to the piezo motor, this is now possible during running operation, which also significantly shortens the maintenance time for calibrating the system.
"PiezoMotor offers a broad product range and modular design. We have found many options for both linear and rotating motors with different features from which we can select the appropriate models," Dr. Pärnaste explains. "Furthermore, PiezoMotor has a highly competent team of engineers and they contributed a lot during our product development process."